Antibiotic Nanozyme
Antibiotic Nanozymes Coassembled by Antibiotics and Hemin
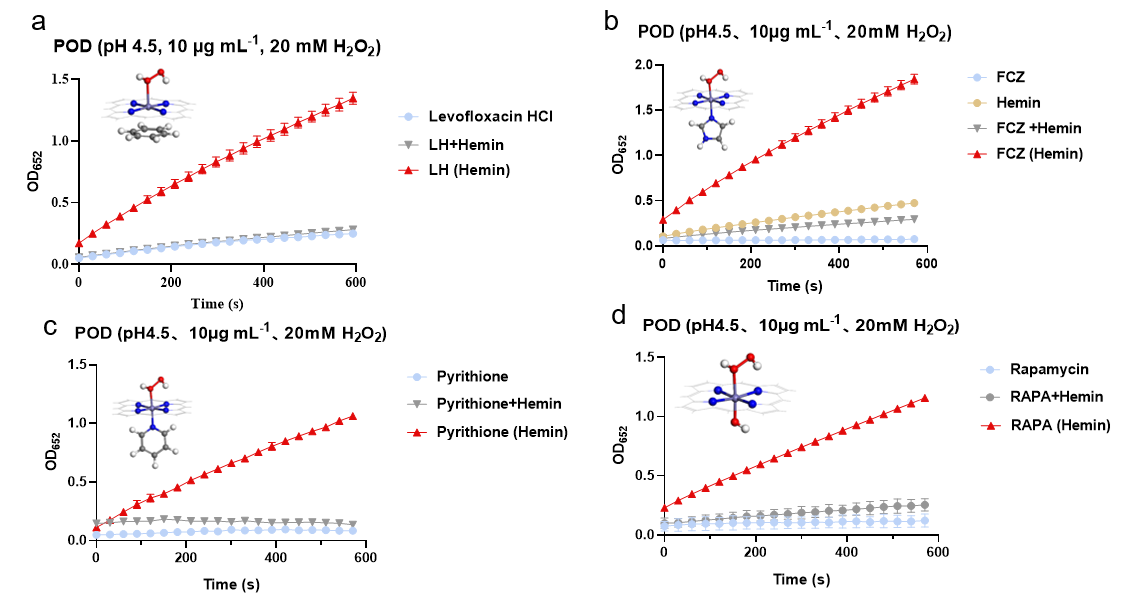
Peroxidase(POD)-like Activity Assay
We systematically evaluated the POD activity of nanozymes formed by the conjugation of various antibiotics with hemin. The results demonstrated markedly enhanced POD activity across all tested nanozymes, validating the broad applicability and efficacy of this assembly strategy for multiple antibiotic classes.
In Vitro Antimicrobial Activity
Antibacterial efficacy of the synthesized nanozymes was evaluated to determine whether the assembly strategy enhances bactericidal activity and broad-spectrum applicability. All antibiotic–hemin co-assemblies exhibited significantly stronger antimicrobial effects compared to their individual components.
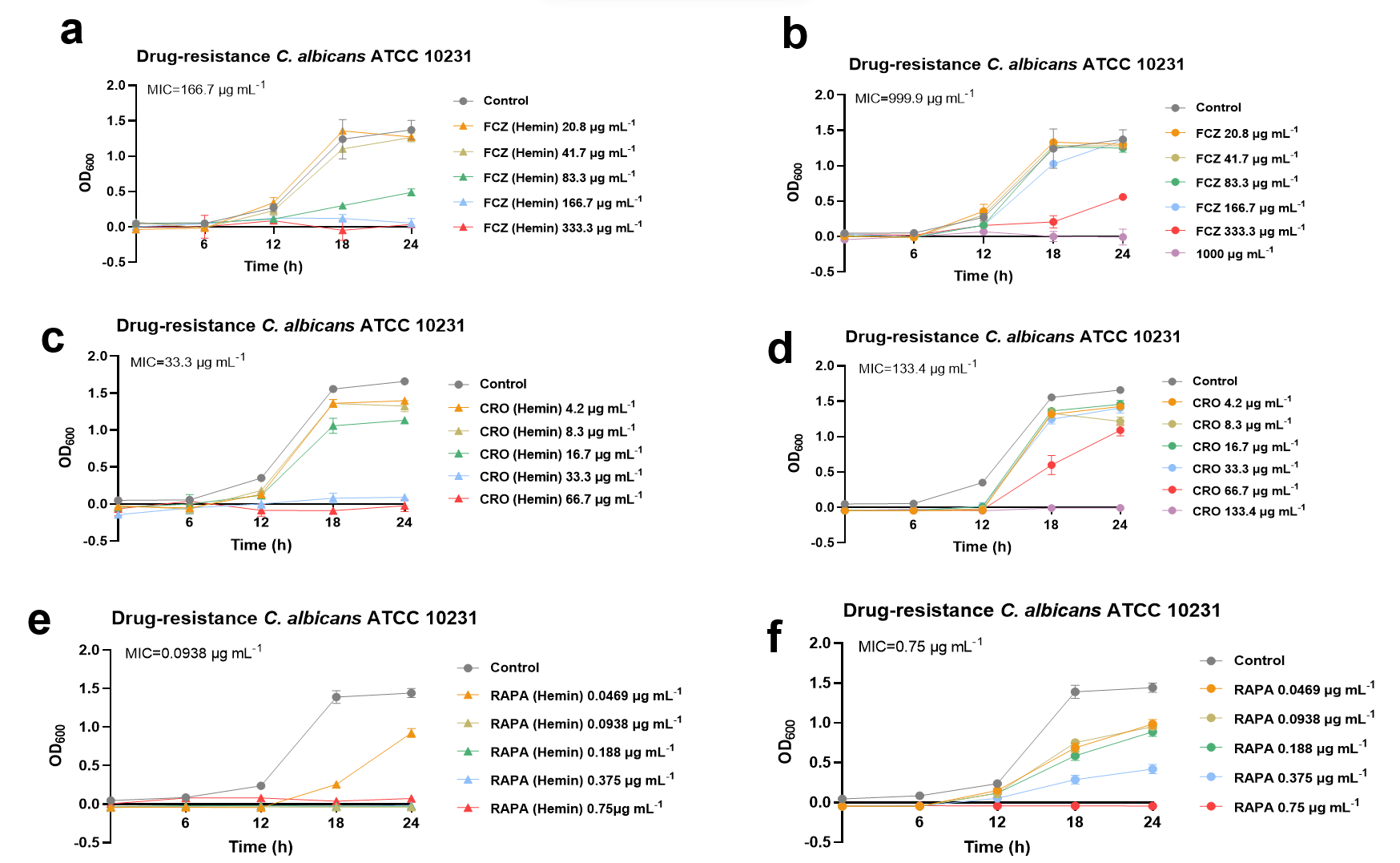
The synergistic effect of ferrous protoporphyrin in promoting the anti-MRSA activity of gentamicin (GM)
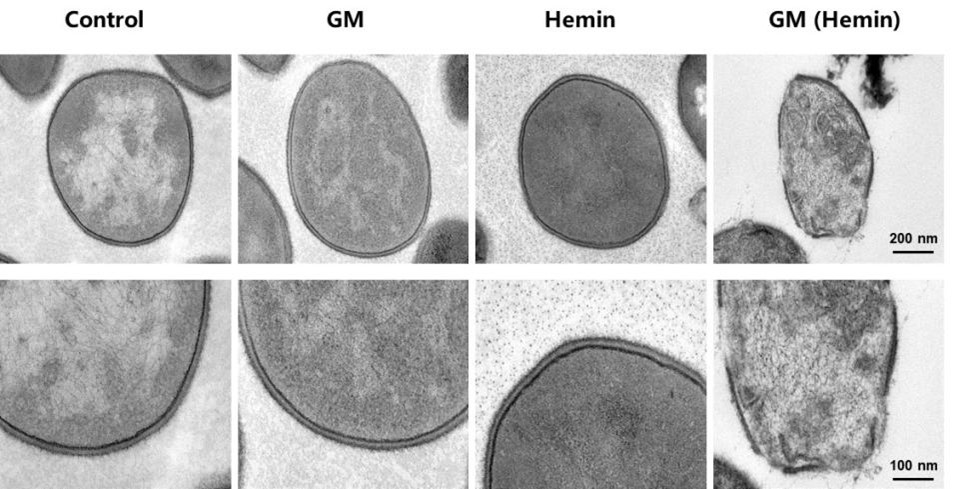
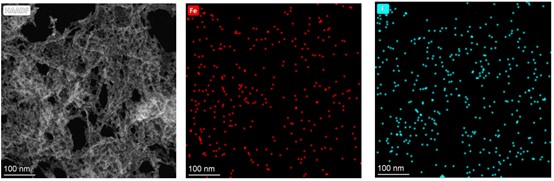
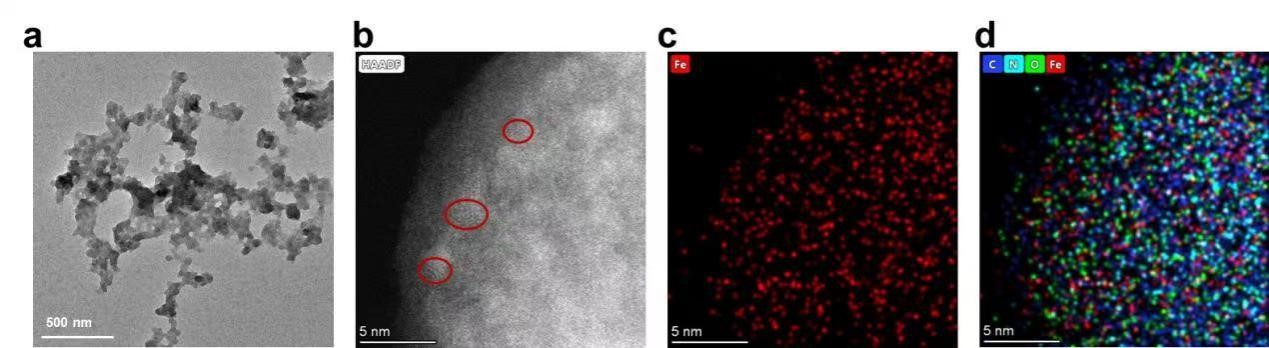
TEM Characterization of GM (Hemin)
TEM revealed a cross-linked spherical nanostructure for the GM (Hemin) (Fig. 8a). High-angle annular dark-field imaging (Fig. 8b) showed uniform iron distribution at 5 nm resolution, while energy-dispersive X-ray spectroscopy(EDS) (Fig.8c,d) confirmed homogeneous distribution of Fe, C, N, and O elements throughout the assembly, verifying successful incorporation of hemin-derived iron.
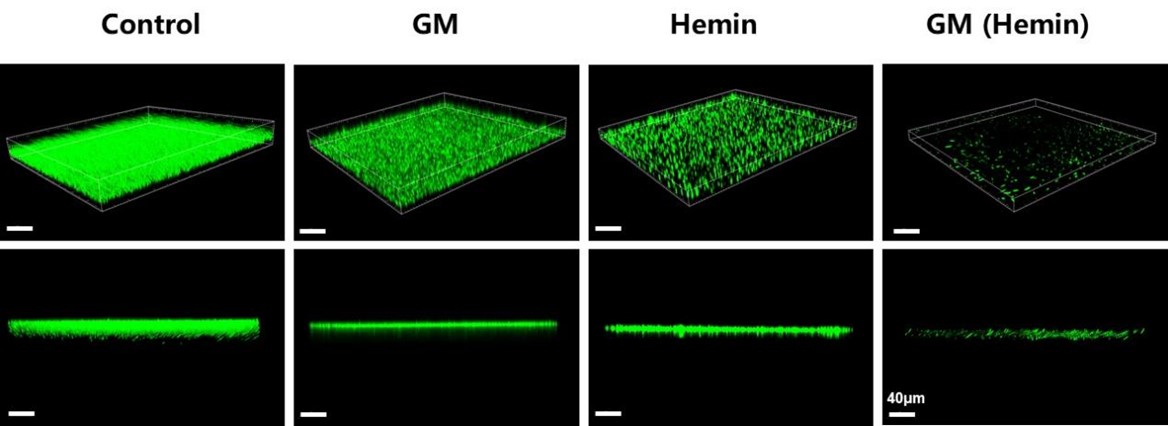
CLSM for Biofilm Eradication Assessment
Three-dimensional confocal laser scanning microscopy revealed significant reduction in bacterial density and biofilm thickness following gentamicin nanozyme treatment, providing direct visual evidence of biofilm disruption capability consistent with quantitative plate counting results.