Where Does Our Inspiration Come From?
In ancient wooden structures, the mortise-and-tenon joint has been widely utilized owing to its remarkable mechanical strength, durability, structural simplicity, and aesthetic refinement. A defining characteristic of this joint system is its interlocking mechanism, in which one component features a projecting tenon and the other a precisely shaped mortise. Upon assembly, the tenon fits tightly into the mortise, forming a secure and stable connection without reliance on external fasteners.
Inspired by this elegant architecture design, we propose a strategy to control the spatial arrangement of functional units through cooperative coordinated co-assembly, aiming to construct a novel nanozyme system for combating antimicrobial resistance.
How Do We Design Antibiotic Nanozymes?
Screening of Targeted Antibiotics
Several representative antibiotics were selected for this study, including gentamicin (GM), which exhibits efficacy against both Gram-negative and Gram-positive bacteria, and fluconazole (FCZ), a first-line clinical antifungal agent used in combination with hemin.
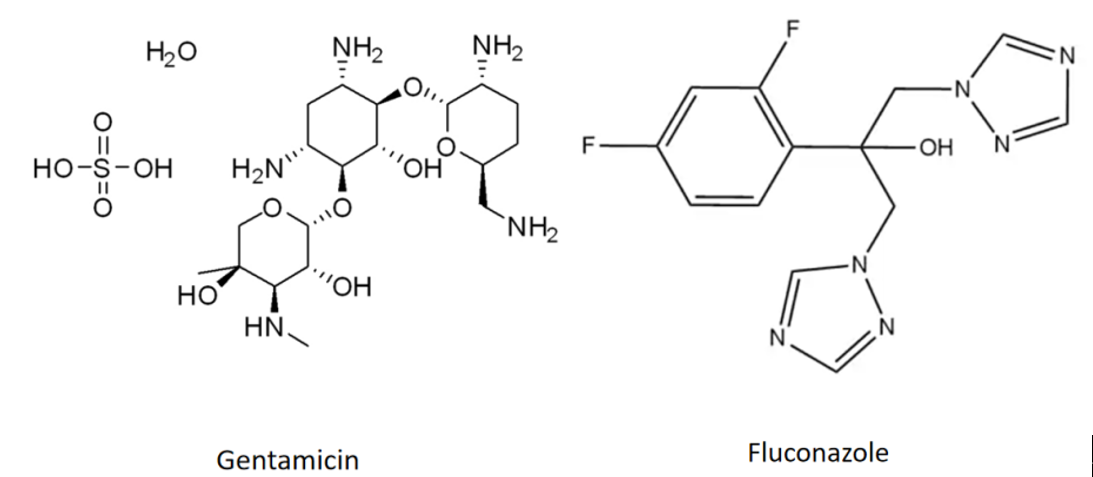
Additionally, over 1,700 FDA-approved drugs were screened through virtual screening to identify potential antibiotics targeting glucan in Candida albicans. Notable candidates identified include rapamycin (RAPA), previously reported by a Nobel Prize-winning scientist to effectively inhibit fungal growth[1], and ceftriaxone sodium (CRO).
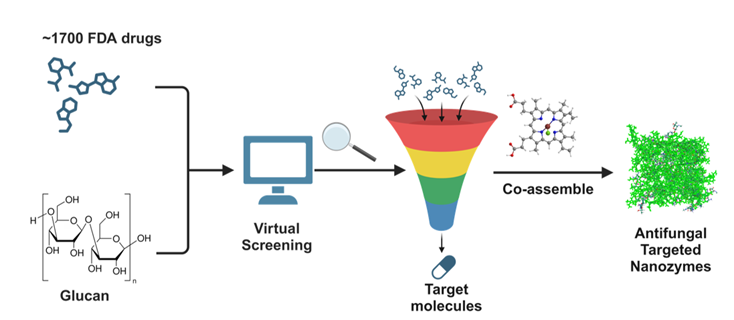
Selection of the adjuvant
Hemin, an essential molecule in the human body, serves as a cofactor in numerous enzymatic reactions and plays a pivotal role in the transport of gaseous molecules[2]. In cytochromes (cyt), heme undergoes axial coordination with specific amino acid residues. These axial ligands are crucial for modulating the structural and functional properties of hemoproteins, including redox potential, electronic configuration, spin state, electron transfer rate, and catalytic activity[3].
Co-assembly Process of Antibiotic Nanozymes
Antibiotics contain diverse functional groups, such as imidazole, amino, hydroxyl, benzene rings, and pyridine moieties, which can effectively substitute the chlorine atom in chlorinated heme and function as axial ligands to the Fe-N4 plane, thus forming a five-coordinate complex[4].
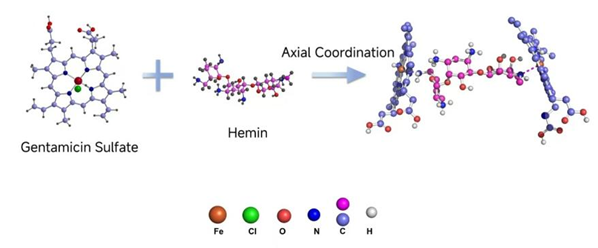
The Universality of the Assembly Strategy for Antibiotic Nanozymes
Antibiotic nanozymes can be engineered by employing various antibiotics, and the integration of different adjuvants may broaden their applicability, thereby improving functional versatility.
What Are the Functions of Antibiotic Nanozymes?
So far, we have successfully constructed a series of antibiotic nanozymes through the axial coordination between heme and different representative antibiotics, such as GM and FCZ, designated as GM(Hemin) and FCZ(Hemin), respectively. Notably, each nanozyme exhibits a consistent capacity to reverse antimicrobial resistance.
Taking GM(Hemin) as an example, this assembly effectively eradicates biofilms by degrading glutathione (GSH) within the biofilm matrix. Subsequently, by leveraging its peroxidase (POD)-like activity, GM(Hemin) generates reactive oxygen species (ROS), which promote bacterial lipid peroxidation and compromise membrane integrity. Additionally, it could induce intracellular ROS accumulation, thereby enhancing the efficacy of co-administered antibiotics. The antibacterial strategy activates ferroptosis-like pathways, resulting in significant suppression of bacterial viability.
Similarly, FCZ(Hemin) could function as a ferroptosis inducer. This process enhances fungal ferroptosis, thereby improving the antimicrobial efficacy of FCZ. The free radicals generated by FCZ(Hemin)-catalyzed reactions further increase intracellular ROS levels, leading to amplified ferroptosis and increased antibiotic uptake in drug-resistant fungal strains.
References
[1] Liu, N.N., et al., Phosphate is the third nutrient monitored by TOR in and provides a target for fungal-specific indirect TOR inhibition. Proceedings of the National Academy of Sciences of the United States of America, 2017. 114(24): p. 6346-6351.
[2] Jian, T., et al., Highly stable and tunable peptoid/hemin enzymatic mimetics with natural peroxidase-like activities. Nat Commun, 2022. 13(1): p. 3025.
[3] Lu, Y., S.M. Berry, and T.D. Pfister, Engineering Novel Metalloproteins: Design of Metal-Binding Sites into Native Protein Scaffolds. Chemical Reviews, 2001. 101(10): p. 3047-3080.
[4] Zhang, R.F., et al., Edge-Site Engineering of Defective Fe-N Nanozymes with Boosted Catalase-Like Performance for Retinal Vasculopathies. Advanced Materials, 2022. 34(39).